When using CRISPR genome editing in stem cells, it’s far easier to break a gene with indels than to fix it with HDR. This manifests in an interesting way. If you...
READ MORE
When using CRISPR genome editing in stem cells, it’s far easier to break a gene with indels than to fix it with HDR. This manifests in an interesting way. If you monitor a “CD34+” population of hematopoietic stem and progenitor cells (HSPCs) from the bone marrow, indels start high and stay high but HDR alleles are lost over time. Why do these different genetic outcomes differ over time? Is HDR bad for the long-term stem cells? Or is editing in the CD34+ population actually heterogeneous, and different cells get different alleles? New work from postdoc Jenny Shin in the lab, out in Cell reports, both answers this question and finds a way to fix the problem.
Jenny and collaborators used a powerful combination of immunophenotyping, next generation sequencing, and single-cell RNA-sequencing to investigate and reprogram genome editing outcomes in subpopulations of adult human CD34+ HSPCs. These HSPCs are actually several different types of cells, including more differentiated progenitors that cycle and very “stemmy” long-term HSCs that are quiescent. The team found that there is a dramatic tension between HDR and quiescence in LT-HSCs. Quiescent stem-enriched cells utilize NHEJ and exhibit almost no HDR. By contrast, non-quiescent cells with the same immunophenotype utilize both NHEJ and HDR. Quiescence is critical for engraftment and stem cell maintenance, so it was now clear that all cells in the CD34+ population get indels and the cycling progenitors were getting HDR alleles, but the quiescent LT-HSCs weren’t doing HDR.
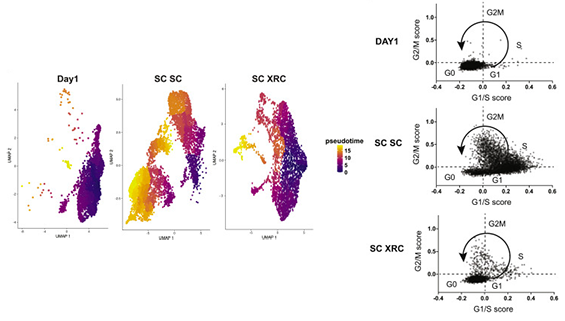 Jenny then had a very creative idea. She asked if a previously reported small molecule cocktail, “XRC”, that maintains quiescence could be used after the fact to re-quiesce LT-HSCs. Using this new strategy and good timing, she found a way to get LT-HSCs with high levels of HDR by briefly allowing them to cycle during editing, and then inducing quiescence later on. This yielded a 6-fold increase in the HDR/NHEJ ratio in quiescent stem cells ex vivo and during long-term engraftment in mouse experiments. The re-quiescence strategy might in future be combined with engineered Cas9-geminin constructs that reduce NHEJ, further tipping the balance towards HDR. Jenny’s results highlight the tradeoffs between editing and fundamental cellular physiology and suggests strategies to manipulate quiescent cells for research and therapeutic genome editing.
Jenny then had a very creative idea. She asked if a previously reported small molecule cocktail, “XRC”, that maintains quiescence could be used after the fact to re-quiesce LT-HSCs. Using this new strategy and good timing, she found a way to get LT-HSCs with high levels of HDR by briefly allowing them to cycle during editing, and then inducing quiescence later on. This yielded a 6-fold increase in the HDR/NHEJ ratio in quiescent stem cells ex vivo and during long-term engraftment in mouse experiments. The re-quiescence strategy might in future be combined with engineered Cas9-geminin constructs that reduce NHEJ, further tipping the balance towards HDR. Jenny’s results highlight the tradeoffs between editing and fundamental cellular physiology and suggests strategies to manipulate quiescent cells for research and therapeutic genome editing.
X Close

 Jenny then had a very creative idea. She asked if a previously reported
Jenny then had a very creative idea. She asked if a previously reported 