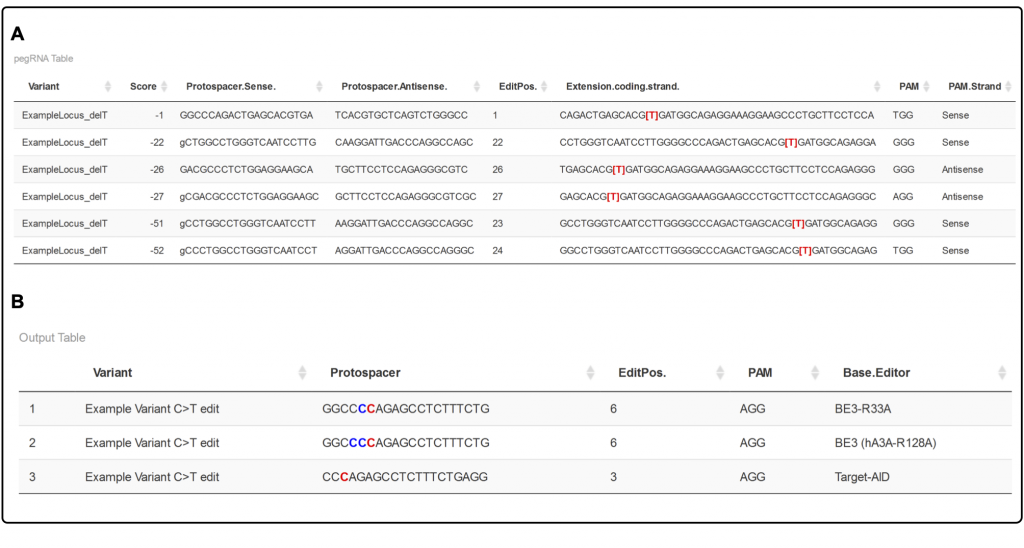
C-TERMINAL AMIDES FUNCTION AS SIGNALS FOR PROTEIN DEGRADATION- PUBLISHED IN NATURE
Proteins are essential building blocks of life, but they can become toxic to our cells if damaged, for example under oxidative stress. In turn, human cells ...
Proteins are essential building blocks of life, but they can become toxic to our cells if damaged, for example under oxidative stress. In turn, human cells selectively remove damaged proteins to maintain a healthy proteome. But how can cells identify individual damaged proteins among thousands of intact ones? An old hypothesis states that cells scan the proteome for chemical modifications that occur, for example, when proteins break. Are you interested in learning more about how cells combat chemical protein damage?
Have a look at our newest research breakthrough, led by postdoc Matthias Muhar in a collaboration with Jakob Farnung from the Bode group (D-CHAB) as well as with Jessberger group (UZH), Jinek group (UZH), Mann group (Max Plank Institute of Biochemistry) Germany and Schulman group (Max Plank Institute of Biochemistry, Germany).
In this study, using a semi-synthetic chemical biology approach coupled to cellular assays, we found that C-terminal amide-bearing proteins (CTAPs) are rapidly cleared from human cells.
To identify the cellular machinery underlying CTAP clearance, we utilized a genome-wide CRISPR screen for genes that are responsible for specific degradation of C-terminally amidated proteins. We identified SCF–FBXO31 ubiquitin ligase as a key reader of C-terminal amides, marking CTAPs for proteasomal degradation. With a conserved binding pocket, FBXO31 exhibits remarkable selectivity, binding C-terminal peptides with amides while excluding non-modified proteins. This mechanism allows cell to remove CTAPs, which form when proteins break under under oxidative stress. Intriguingly, a human mutation linked to neurodevelopmental disorders alters FBXO31’s substrate recognition, leading to toxicity. These findings suggest CTAPs may represent a new class of modified amino acid degrons (MAADs) that mark proteins for removal by reader proteins and downstream effectors, offering insights into selective surveillance of chemically damaged proteins.
In conclusion, this research uncovered new signals for protein clearance and advanced our understanding of cellular protein quality control.
For more info check out our new paper in Nature!







 Jenny then had a very creative idea. She asked if a previously reported
Jenny then had a very creative idea. She asked if a previously reported 

