Better comms between academia and industry
As announced at our Grand Opening, we've launched into a
As announced at our Grand Opening, we've launched into a big collaboration with AstraZeneca to use CRISPR for basic research aimed at impacting human health. Just as important, we've also just started a very exciting partnership with Agilent, providing access to technologies we've previously only dreamed about. So what are these collaborations all about? What do we hope to do? Why are we working with companies at all?
I think this is all about two-way communication. From my time at Genentech, I know that industry certainly pays a lot of attention to what's going on in academia (below I'm mostly talking about academics trying to directly make an impact for human health). But there's also frustration, because some ideas are really great and then plow themselves right into the ground during execution. For example, a super-cool proof of concept that will has no chance of impacting patients because the [assay|intervention|etc] is unnecessarily jury-rigged or the [results|compounds|cells] weren't discussed with anyone who might spend the next 12+ years to turn the idea into a therapy. Derek Lowe has any number of posts about these kinds of papers, and has strong words to say about most academic small molecule screens. In these cases, industry wistfully sighs, "If only they had done X, Y, or Z, we would be so excited to work with them to make it go further!" But unfortunately, there's very little bandwidth for those kinds of long-term gambles in an industry lab (Genentech was an exception, thankfully for me). In this way, some academic work that aims to help people instead just nets the authors a cool paper and then dwindles away. This is incredibly frustrating for both sides, since everyone truly wants to make a positive impact on the world and
That's exactly what we want to avoid, and we're going to do it with two-way communication and tight collaboration. What high-risk (e.g. academic) research needs to be done in the medium term to help better therapies get to patients faster? What are new and emerging areas where breakthrough science meets potential to impact health? What are the long-term moonshots that might actually get picked up and turned into a world-changing therapy if successful? Both academic and industry scientists have strong feelings about these questions, but all too often we exist in the monoculture of our immediate environment and fail to really communicate with one another.
The IGI's collaborations with Agilent and AstraZeneca represents our first steps in the road to reversing this trend. We'll be doing basic research together with groups from both companies, in a truly collaborative mode. We hope to both to give them insight into new and great scientific discoveries on the horizon, and also to get their insight into areas where basic research now can have a dramatic impact in the long term. I think that through collaborations with industry groups we can make a very positive impact in the fight against many diseases (stay tuned for more on the science!); it's going to be an exciting time!

 Cas9 bound to sgRNA and DNA target
Cas9 bound to sgRNA and DNA target HnH domain removed (sgRNA in orange, DNA in blue)
HnH domain removed (sgRNA in orange, DNA in blue) The DNA and sgRNA can be removed from the model (PAM in green)
The DNA and sgRNA can be removed from the model (PAM in green) The DNA strands can even be separated at the actual Cas9 cleavage site
The DNA strands can even be separated at the actual Cas9 cleavage site
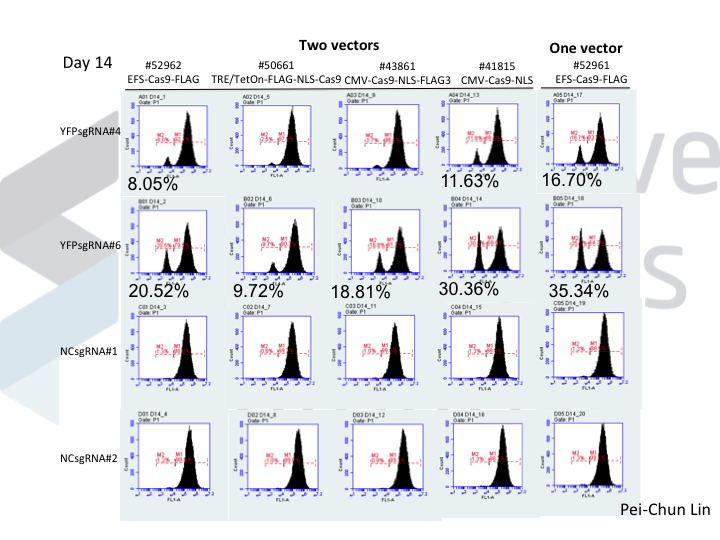
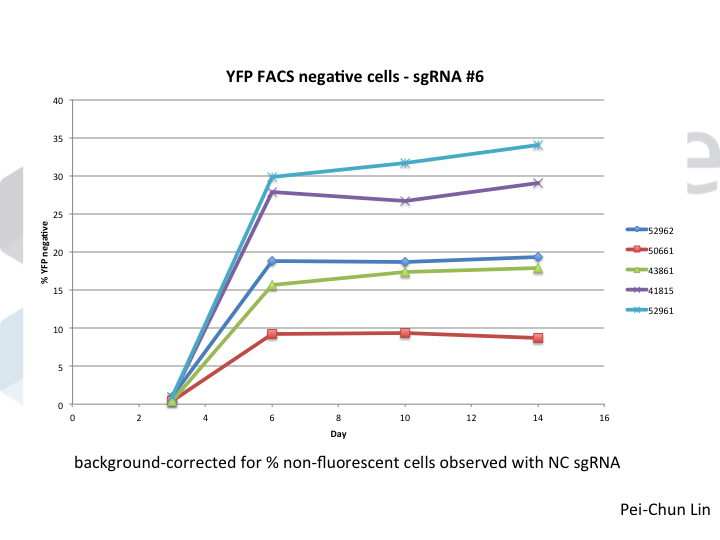


 Welcome to Lena Kobel, who joins the lab as a Cell Line Engineer. Lena has a long history in genome engineering, with previous experience in Martin Jinek’s...
Welcome to Lena Kobel, who joins the lab as a Cell Line Engineer. Lena has a long history in genome engineering, with previous experience in Martin Jinek’s...